Why is Ice Less Dense Than Water
That is why ice floats on water for which we should all be thankful because if water behaved normally many bodies of water would freeze solid in the winter killing all the life within them. One of the similarities is a layered interior but Ceres layers arent as clearly defined.
Part of our research focused on the Vanderford Glacier in East Antarctica.

. There we observed the warm water replacing the colder dense shelf water. The refraction occurs at the boundary and is caused by a change in the speed of the light wave upon crossing the boundary. In some cases for.
Gelato also contains less air than American ice cream that helps keep it dense fluid and creamy. Inflatable sandbag uses a degradable polymer to absorbs water then block it. Where ρ is the density m is the mass and V is the volume.
For this reason water is one of the few substances that is actually less dense in solid form than in the liquid state dropping from 1000 to 917 kilograms per cubic metre. It is this open solid structure that causes ice to be less dense than liquid water. The density of water once again is a special case.
Water is called the universal solvent because more substances dissolve in water than in any other chemical. Water ice wind living organisms and gravity break rocks soils and sediments into smaller particles and move them around. Sunshine the ground heats the air just above it.
In practical terms density is the weight of a substance for a specific volume. This then causes the ice shelves to. Ice is less dense than liquid water which is why your ice cubes float in your glass.
When given warm water and food sugar gluten etc causes gas to be released in the dough. The solid form of water ice is less dense than its liquid form. The gas is.
Delivered as a flat one-pound sack they absorb up to 45 pounds of fresh water in five minutes forming a dense gel that blocks and redirects water while forming to each other or adjacent structures for a tighter fit than traditional sandbags. Water is most dense at 39F and as it cools or warms from this temperature the water expands slightly. Density volumetric mass density or specific mass is the substances mass per unit of volumeThe symbol most often used for density is ρ the lower case Greek letter rho although the Latin letter D can also be used.
Regular hexagonal ice is also less dense than liquid waterupon freezing the density of water decreases by about 9. Human activities affect Earths systems and their interactions at its surface. That warmed air starts to rise because when warm it is lighter and less dense than the air around it.
It is the reason why ice floats rather than sinking so that during the winter it develops as a sheet on the surface of lakes and. It contains 11188 percent hydrogen and 88812 percent oxygen by weight. As the ice shell distorts and flexes from tidal forces warmer and less-dense ice would rise carrying the ocean samples to the surface where.
As you might expect water density is an important water measurement. The atmosphere is the superhighway in the sky that moves water everywhere over the Earth. Refraction is the bending of the path of a light wave as it passes from one material to another material.
As it rises its pressure and temperature drop causing water vapor to condense. Instead one of the liquids is. Because theres less butterfat coating your palate gelatos flavors tend to taste more intense.
This means that ice is slightly less dense than cold water which is why ice floats on the surface of bodies of water. Ceres is more similar to the terrestrial planets Mercury Venus Earth and Mars than its asteroid neighbors but it is much less dense. The movement of warm waters towards East Antarctica is expected to worsen throughout the 21st century further threatening the ice sheets stability.
Imagine how hard would it be to have life in water if every winter the whole water froze. Without this property of water life would probably not be possible. Ice and liquid water at low temperature have comparatively low-density low-energy open lattice structures.
Ice is the solid state of water. The tendency of a ray of light to bend one direction or another is dependent upon whether the light wave speeds up or slows down upon crossing the. So lets take a look.
Water definition a transparent odorless tasteless liquid a compound of hydrogen and oxygen H2O freezing at 32F or 0C and boiling at 212F or 100C that in a more or less impure state constitutes rain oceans lakes rivers etc. Making light fluffy bread or bread that is less dense is mostly a matter of using the right ingredients and to some extent the right process. Ice cream is an emulsiona combination of two liquids that dont normally mix together.
Why this matters to marine life. Water at the Earths surface evaporates into water vapor which rises up into the sky to become part of a cloud which will float off with the winds eventually releasing water back to Earth as precipitation. The main ingredients to accomplish this are 1 bread flour 2 instant yeast 3 dough enhancers.
Since ice is less dense than liquid water it floats in liquid water and prevents the freezing of the whole water body. Waters density maximum is a product of the same phenomenon. Due to the high fat content of ice cream however and because fat is less dense than water any ice cream will always be less dense than any aqueous solution otherwise you would not be able to make root beer floats.
Its much more efficient than gasoline but it comes with a premium price tag. This has to do with the polarity of each water molecule. This helps water.
Mathematically density is defined as mass divided by volume. From ground-based telescopes scientists knew that Europas surface is mostly water ice and scientists have found strong evidence that beneath the ice crust is an ocean of liquid water or slushy ice. The lighter and less dense continents are embedded in heavier and denser upper-mantle rocks and together they make up the moving.
The hydrogen side of each water H 2 O molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge. The density of water is roughly 1 gram per milliliter but this changes with temperature or if there are substances dissolved in it. The water and ice that make up clouds travels into the sky within air as water vapor the gas form of water.
These peculiar effects are due to the highly directional bonding of water molecules via the hydrogen bonds. Although floating ice does not change sea level when it melts any more than a glass of water will overflow when the ice cubes in it melt scientists became concerned that the collapse could. Diesel is an energy-dense fuel that makes it a great option for semis and large trucks pulling heavy loads.
The less-dense freshwater moves along quickly near the surface of the ocean and traps relatively warm ocean saltwater against the underside of the ice shelves. This section of the Water Science School discusses the Earths natural. With the increased price in fuel lately many drivers wonder why diesel is more expensive than gas.
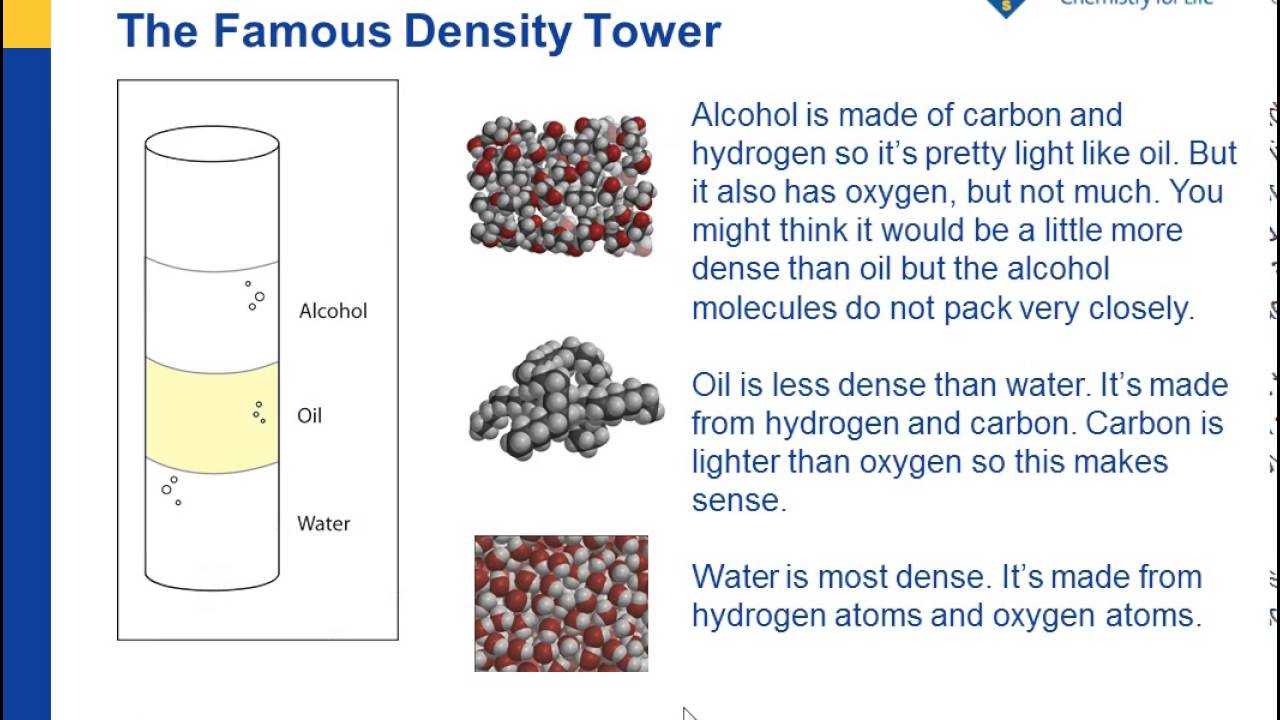
Density Sink And Float For Liquids Chapter 3 Density Middle School Chemistry Middle School Chemistry Teaching Chemistry Chemistry

Ice Floats Because Solid Water Is Less Dense Than Liquid Water Arctic Landscape Landscape Outdoor

Because Of Hydrogen Bonding Water Is Less Dense As A Solid Than It Is As A Liquid Consequently Ice Floats Language Chemistry Education

Scientific Explanation Of Why Ice Floats Elementary Science Hydrogen Bond Floating

No comments for "Why is Ice Less Dense Than Water"
Post a Comment